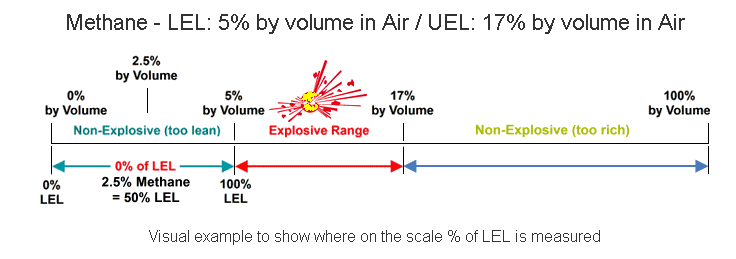
The lower explosive limit (LEL) is the minimal amount of concentration needed of a particular chemical to cause an explosion. The LEL is determined empirically for each pure chemical and air mixture at a given temperature. If more than than one chemical is dispersed in the air, as is normally the case, then LeChatelier’s mixing rule can be applied to get the cumulative LEL for the mixture. Concentrations lower than the Lower Explosive Limit are 'too lean' to burn; those above the Upper Explosive Limit (UEL) are too rich to burn.The amount of gas present is specified as a percentage (%) of LEL. Zero percent Lower Explosive Limit (0% LEL) denotes a combustible gas-free atmosphere. One hundred percent lower explosive limit (100% LEL) denotes an atmosphere in which gas is at its lower flammable limit. The relationship between percent LEL and percent by volume differs from gas to gas.The example below demonstrates the flammability of Methane (Natural Gas) in Air. In concentrations of 0-5% Methane in air, the mixture is too lean to ignite or burn. Methane concentrations between 5% and 17% will support ignition and are considered highly flammable. At levels above 17%, the atmosphere is too rich for the methane to ignite.