Nitric Oxide (NO) Calibration Gas
Nitrous Oxide
Nitrous Oxide (N2O), not to be confused with Nitric Oxide (NO), is a colorless gas with a faintly sweet, pleasant odor and a slight metallic taste. This gas is produced both in nature and by human sources. Important natural sources include the oceans and soils that are located under natural vegetation. The most substantial source (about 67%) of nitrous oxide emissions are derived from the agriculture industry. Human waste is also a significant origin of Nitrous Oxide emissions. N2O is commonly known as "laughing gas", but it does exhibit some characteristics that are no laughing matter. One of the more severe effects associated with exposure to N2O is its possible Teratogenic effects (cause birth defects in embryos and fetuses or infertility) and its direct role in creating an oxygen deficient atmosphere.
Also Known As: Laughing Gas, Dinitrogen monoxide, Sweet Air, Hyponitrous oxide, Nitrous
Nitrous Oxide (NO2): Chemical Dangers
Violent reactions will occur if contact is made with phosphine, hydrazine, and aluminum among many other substances. Nitrous Oxide is not flammable but will enhance the combustion of other substances. N2O has oxidizing properties which means it will intensify fires. This Gas can be harmful to workers depending the concentration and duration of exposure. Nitrous Oxide is considered to be a reactive gas.
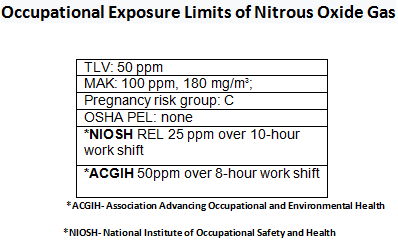
Health Effects Associated With Nitrous Oxide Exposure
Other than its infamous euphoric and hallucinogenic effects, there are several other effects that are directly associated with exposure to Nitrous Oxide. Despite its many positive uses, N2O can be very dangerous as it can cause short-term decreases in your ability to make sound, logical and life sustaining decisions, which could put your safety at risk in occupational settings. Oxygen deprivation can occur if Nitrous Oxide is inhaled without the proper amount of O2 present. This could lead to a drastic drop in blood pressure and fainting. Some of the more long term effects caused by exposure to N2O include Vitamin B deficiency, damage to the nervous system causing numbness and tingling feelings, and irreversible birth defects to embryos and fetuses. Frostbite (liquid exposure) and asphyxiation can also occur in the right environments.
Industrial Applications for Nitrous Oxide (N2O)
Nitrous Oxide is primarily found in nature from the emissions from agriculture, oceans, and soils under natural vegetation. The use of Nitrous Oxide can be seen in numerous industries. Its most popular use is in the medical field as an anesthetic or analgesic, typically in a dental office or in surgical rooms. N2O is also utilized as foaming agent in whipped cream, quadrupling the volume and making it extra "fluffy". Like fast cars? Well, Nitrous Oxide is also the key component in a Nitrous Oxide engine, which allows the increase of the internal combustion engine's power output by allowing fuel to be burned at a much higher rate.
Handling and Storage Tips for Compressed Nitrous Oxide Gas
Store cylinders a well-ventilated, temperature controlled area
Secure properly, protected from weather
Store upright, ensuring valve protection caps are in place
Full and empty containers should be segregated
Do not drag, slide or drop compressed gas cylinders
Due to possibility of theft, Nitrous Oxide cylinders should be stored in a controlled environment.
